Is it safe to take Amoxicillin 500mg during pregnancy?
Pregnancy is a special physiological condition where drug treatment presents a special concern, keeping in mind the safety of the mother and the kid. The fetus is highly vulnerable to congenital disabilities between 3rd week and 8th week after fertilization, which is the period of organogenesis. All major organs start developing during this period. Drugs reaching the fetus during this stage may cause a miscarriage, an obvious birth defect, or a permanent but subtle defect that is noticed later in life.
At the 9th week, the embryo is referred to as a fetus, and development during this time is primarily maturation and growth. Drug exposure during this period is not associated with major congenital malformations, but they may alter the growth and function of normally formed organs and tissues.
Total avoidance of pharmacological treatment during this period is an infeasible option and even downright dangerous because some pregnancy conditions require medical interventions that, when not conducted, can pose risks for both the mother and the baby. E.g., pre-existing conditions like asthma or hypertension with new pregnancy-induced medical problems like infections, fetal problems, and miscarriage require pharmacological intervention.
Urinary tract and vaginal infections are a few common complications in pregnant women and necessitate antibiotics. Antibiotics are also administered in pregnant women to prevent infections like Group B Strep in the fetus/unborn baby. Amoxicillin 500mg is an antibiotic very similar to penicillin and is administered to counter urinary and respiratory infections in pregnant ladies.
Amoxicillin is an amino-penicillin created by adding an extra amino group to fight antibiotic resistance effectively. This imparts an efficient profile and enables it to cover a wide variety of gram-positive and gram-negative bacteria compared to penicillin, especially drug-resistant species Streptococcus species and has improved coverage of Listeria monocytogenes and Enterococcus spp. It also covers Haemophilus influenza, some Escherichia coli, Actinomyces spp., Clostridium species, Salmonella spp., Shigella spp., and Corynebacteria species.
But there are mixed reports regarding its safety during pregnancy.
What do studies say
To evaluate the safety of Amoxicillin in pregnancy conditions, case reports of 877 infants with cleft lip and with/without cleft palate and 471 infants with only cleft palate were evaluated by researchers in a study. For comparison purposes, 6952 non-malformed infants were included as a control in the study. Mothers were interviewed about demographic, reproductive, and medical factors and details of medication use.
They estimated odds ratios and confidence intervals associated with using Amoxicillin in the first trimester of pregnancy while keeping in mind risk factors for clefts like infections, fevers, and concomitant treatment with other medicines. In the control group, 3% of women had used Amoxicillin in the first trimester. Maternal use of Amoxicillin was associated with an increased risk of Cleft lip with or without a palette with an increased odds ratio during the third gestational month of use of medicine. Risks were not elevated for the use of other penicillins or cephalosporins. For cleft palate conditions, the odds ratio was less in the first trimester and significantly increased in the third gestational month of pregnancy.
The study concluded that Amoxicillin use in early pregnancy might be associated with an increased risk of oral clefts. Clinicians, pregnant women, and public-health officials need information on the potential teratogenic effects of Amoxicillin to make risk-benefit judgments on its use early in pregnancy.
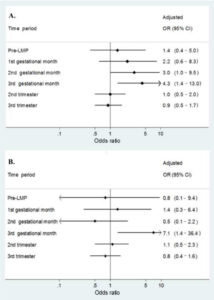
Image credits- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3729019/
While the study mentioned above contradicted the use of Amoxicillin, another study showed positive results in favor of Amoxicillin safety.
The study included 101615 pregnancies, of which 6919 were exposed to Amoxicillin; 1045 pregnant subjects were subjected to Amoxicillin only, and 6041 subjects were administered Clavulanic acid, a beta-lactamase inhibitor that works by preventing bacteria from destroying Amoxicillin. In data variables, no significant association was found between first‐trimester exposure to Amoxicillin or Clavulanic Acid and major malformations in general or for major malformations according to organ systems. Overall, 6166 (6.1%) pregnancies resulted in major malformations: 2384 (2.3%) with cardiovascular malformations, 180 (0.2%) with central nervous system malformations, 825 (0.8%) with genitourinary malformations, 319 (0.3%) with gastrointestinal malformations, 131 (0.1%) with cleft palate and spina bifida occurred in 76 (0.1%) pregnancies.
No dose-response relationship was found between exposure in terms of the defined daily dose and major malformations. The study proved that exposure to Amoxicillin and Clavulanic Acid during the first trimester of pregnancy was not associated with major malformations in general or according to organ systems.
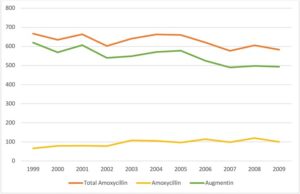
Image credit- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6955403/
What does the CDC say about the use of Amoxicillin during pregnancy?
In 2012, the Centers for Disease Control and Prevention (CDC) partnered with the Association of Maternal and Child Health Programs to discuss the prevention and treatment of anthrax in pregnant, postpartum, and lactating women. According to published data, high maternal and fetal death rates have been reported in pregnant and postpartum women exposed to Bacillus anthracis, a gram-positive and rod-shaped bacterium transmitted from animals to humans.
According to the CDC, ciprofloxacin (500 mg, orally, every 12 hours for 60 days) is the preferred treatment for prophylaxis of inhalational anthrax in asymptomatic pregnant and lactating women exposed to Bacillus anthracis. If ciprofloxacin is unavailable or not tolerated, CDC recommends using other antibacterial drugs for prophylaxis of inhalational anthrax, such as doxycycline (100 mg every 12 hours). Amoxicillin does not have an FDA-approved indication for anthrax prophylaxis or treatment; however, if the strain of Bacillus anthracis is penicillin-susceptible, prophylactic therapy with Amoxicillin may be considered, according to the CDC.
Amoxicillin is one of the most commonly used antibiotics in primary care, with its popularity gradually increasing. In 2004, Amoxicillin was prescribed over 29 million times. In 2018, the number of amoxicillin prescriptions increased to over 31 million. Unfortunately, its use in pregnancy is debatable, with mixed results from studies conducted to evaluate its efficacy in pregnant ladies. More concerning is the misuse of Amoxicillin through self-medication, leading to bacterial resistance to the medicine.
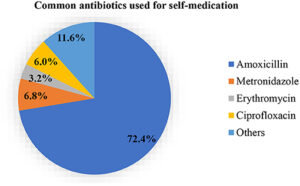
Image credit- https://www.frontiersin.org/articles/10.3389/fpubh.2021.706290/full
What does FDA say about Amoxicillin?
In 1979 the FDA or Food and Drug Administration developed a system that determines the teratogenic risk of drugs by considering the quality of data from animal and human studies. FDA classifies various drugs used in pregnancy into five categories: A, B, C, D, and X. Category A is considered the safest category, and category X is contraindicated in pregnancy. Amoxicillin is assigned pregnancy category B; if there is a clinical need for the drug, it is considered safe to use.
Conclusion
If a woman takes Amoxicillin before realizing that she is pregnant, there is likely nothing to worry about in terms of fetal health and safety. The health and development of a fetus is largely dependent on its mother, so it’s very important for pregnant women and women of childbearing age to stay as healthy as possible. Sometimes, this may include taking antibiotics like Amoxicillin that can be used in pregnant and breastfeeding women as its physical characteristics, like low-fat solubility and low protein binding, limit its transfer to breast milk. But maternal anaphylaxis in case of allergy to the medication can affect the fetus and lead to neurotoxicity and even death, because of which care should be taken to ascertain the allergic status of pregnant patients before using it.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6955403/
https://www.frontiersin.org/articles/10.3389/fpubh.2021.706290/full
Related Post
Buy Ivermectin
Free Express Delivery (Royal Mail)
Dispatched Within 24 Hours
Delivery Within 3-5 Business Days